Lithium-ion (Li-ion) and lead-acid batteries are both rechargeable batteries, but they differ in their chemistry, performance, and applications. Here are some of the main differences between Li-ion and lead-acid batteries:
- Chemistry: Li-ion batteries use lithium ions to move between the positive and negative electrodes, while lead-acid batteries use lead dioxide and lead plates submerged in an electrolyte solution of sulfuric acid.
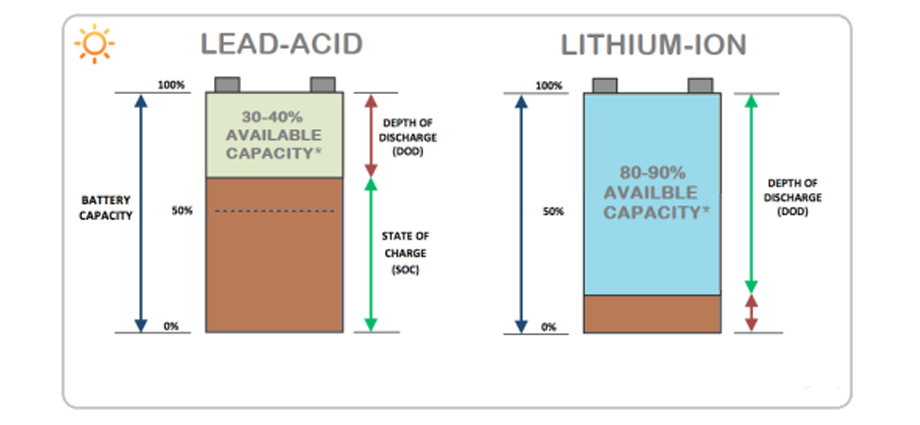
- Energy density: Li-ion batteries have a much higher energy density than lead-acid batteries, which means they can store more energy in a smaller and lighter package. This makes them ideal for use in portable electronic devices and electric vehicles.
- Weight and size: Li-ion batteries are much lighter and smaller than lead-acid batteries for the same energy capacity. This makes them easier to transport and more space-efficient.
- Lifespan: Li-ion batteries generally have a longer lifespan than lead-acid batteries and can be recharged hundreds of times, whereas lead-acid batteries have a limited number of charging cycles.
- Charge rate: Li-ion batteries can be charged more quickly than lead-acid batteries, which can be useful in applications where fast charging is required.
- Cost: Li-ion batteries are generally more expensive than lead-acid batteries, although their cost has been decreasing in recent years as the technology has become more widely adopted.
- Safety: Li-ion batteries are generally considered safer than lead-acid batteries, as they are less likely to leak acid or explode if damaged. However, they do have some safety concerns if not handled properly.
Overall, Li-ion batteries are better suited for applications that require high energy density, low weight and volume, and fast charging, such as in portable electronics and electric vehicles. Lead-acid batteries are better suited for applications that require low cost and high reliability, such as in backup power systems and off-grid solar energy systems.